How to calculate the total chlorine injection quantity for the injection period
We want to know how much of the concentrated chlorine to dilute into the tank that feeds the injection pump.
To do this you need to know:
the flow rate of the fertigation injection pump per hour
the flow rate of the irrigation system per hour
the concentration of the chlorine you will use (g chlorine per liter or %)
the number of hours of injection required
target ppm at the injection point.
The formula to determine the amount of chlorine per hour you need to add:
flow (m³/h) X target ppm at injection / chlorine product concentration (%) X 10 = chlorine (liters/hour)
US Measurements
flow (gpm) X target ppm at injection / chlorine product concentration (%) X 167 = chlorine (gallons/hour)
How to calculate the total chlorine injection quantity according to the injection period
Steps
1. [flow rate of the irrigation system per hour] x [the number of hours of injection required] = the total volume of water to be treated
2. [total volume of water to be treated] x [concentration of chlorine required (the quantity that you calculated above)] = the total volume of chlorine product required
3. [the flow rate of the fertigation injection pump per hour] x [the number of hours of injection required] = the total volume of mixture to be injected by the pump
To prepare a diluted mixture for injection, we need to know how much is chlorine, and how much is water.
4. [total volume of mixture to be injected] – [total volume of chlorine required] = the amount of water that needs to be mixed with the chlorine into the operational tank to be injected

Example
Scenario
• Target ppm at injection: 30 ppm
• Chlorine source concentration: 8% W/V
• Irrigation system flow rate: 60 m³/h (264 gpm)
• Injection duration: 1 hour
• Injection pump: Capacity of 500 liters/hour (132 gal/hour)
Metric Measurements
Calculations for total volume of chlorine to be injected per hour
flow (m³/h) X target ppm at injection / chlorine concentration (%) X 10 = chlorine (liters/hour)
60 (m3/h) x 30 (target ppm) / 8 (% use as full number) X 10 = 22.5 l/h of chlorine to be injected
Calculations for rates of injection and dilution
1. Total volume of water to be treated: 60 m³
2. 500 liters/hour injection capacity of the pump multiplied by the injection period (1 hour) = 500 liters total volume of mixture to be injected
3. Take the total volume of mixture to be injected (500 l) and subtract the chlorine (22.5 liters) = 477.5 liters of water to be in the mixture
4. As such, there will be 22.5 liters of 8% chlorine and 447.5 liters of water that is injected over the one hour of injection
US Measurements
Calculations for total volume of chlorine to be injected per hour
flow (gpm) X target ppm at injection / chlorine concentration (%) X 167 = chlorine (gallons/hour)
260 (gph) x 30 (target ppm) / 8 (% use as full number) X 167 = 5.8 gal/h of chlorine to be injected
Calculations for rates of injection and dilution
1. Total volume of water to be treated: 15,840 gallons
2. 132 gal/hour injection capacity of the pump multiplied by the injection period (1 hour) = 132 gallons total volume of mixture to be injected
3. Take the total volume of mixture to be injected (132 gal) and subtract the chlorine (5.8 gal) = 126.2 gal of water to be in the mixture
4. As such, there will be 5.8 gal of 8% chlorine and 126.2 gal of water that is injected over the one hour of injection
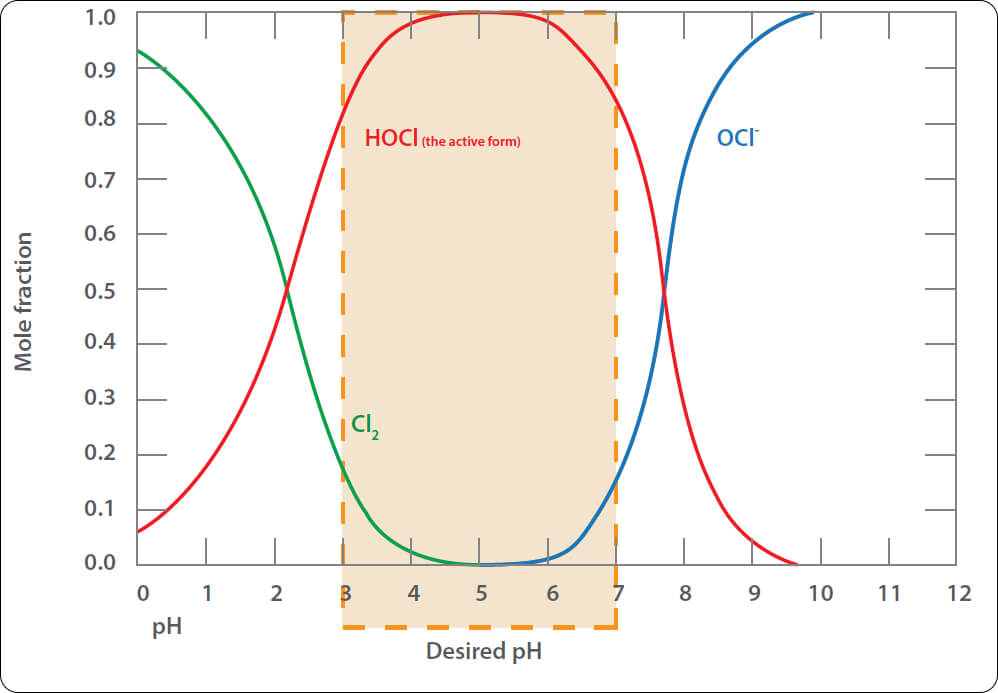
Chlorine reactivity dependent on pH
Keep in mind that the reactivity (effectiveness) of chlorine is highly dependent on the pH of the water.
As discussed earlier, HOCl is a hypochlorite, and Cl2 is inert for the purposes that we require for treatment. Therefore, we want the water to be at a pH that has the maximum HOCl, and the minimum Cl2.
The graph below shows the respective levels at different pH.

The pH of the water will have some effect on the biocidal activity of chlorine. Hypochlorous acid (HOCl) is up to 80 times more effective than the hypochlorite ion, (OCl–). At a pH 5.0, over 90% of the chlorine is present as HOCl. At pH 7.0, 60% is HOCl. At pH 7.5, 40%. For this reason, the water is often treated with acid to lower the pH while injecting chlorine. The acid will also dissolve calcium carbonate deposits.

WARNING: Do not mix acid and concentrated chlorine solutions, as this will rapidly generate
deadly chlorine gas! The two materials must be injected into separate ports in the system.